Titanium
Located in Group 4 and Period 4 in the Periodic Table is the element that is found in almost every living things. This is Titanium.
First discovered in 1791 by William Gregor in Cornwall, Great Britain. Gregor analyzed gun powder-like sand and found a reddish brown clay he could not identify. Four years later in Berlin, renowned chemist Martin Heinrich Klaproth independently discovered the element in rutile. And Titanium got its wonderful name by Martin Heinrich Klaproth after the titans of Greek mythology.
 |
| William Gregor |
Its ores are rutile and ilmenite and they can be found in the Earth's crust and lithosphere as well as body of rocks, water and soils. Titanium can be alloyed with some metals such as iron, aluminium, vanadium and molybdenum to produce strong, light alloys for aircraft, industries, medical instruments and dental instruments.
Atomic Number : 22
Name : Titanium (1791)
Latin Name : Titan (1791)
Electrons per shell : [2, 8, 10, 2]
Discoverer : William Gregor (1791)
Isolator : Martin Heinrich Klaproth
Element's : Atomic Mass : 47.867u
: Density : 4.506 g/cm3
: Type : Transition Metal
Chemical Properties:
- Highly corrosion resistance
- Reacts with Fluorine to form Titanium (III) Fluoride
- Reacts with Chlorine to form Titanium (III) Chloride
- Reacts with Bromine to form Titanium (III) Bromide
- Reacts with Iodine to form Titanium (III) Iodide
- Reacts with Oxygen to form Titanium Dioxide
- ONLY reacts with water after its protective layer (Titanium Oxide) is destroyed and forms Titanium (IV) Oxide and Hydrogen
- Heat up with Nitrogen to form Titanium Nitrate
- Titanium (IV) Chloride is heat up with Sodium or Magnesium to produce Titanium
- Does not react with MOST acids but will react with hot hydrochloric acid to form Titanium (III) Complexes and Hydrogen
- 5 isotopes : 3 of them are :
Neutrons : 22
Electrons : 22
(Half life : 63 years)
(Half life : 63 years)
Titanium - 46 : Protons : 22
Neutrons : 24
Electrons : 22
(Half life : Stable)
Titanium - 48 : Protons : 22
Neutrons : 26
Electrons : 22
(Half life : Stable)
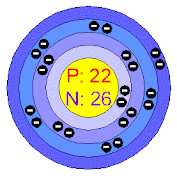 |
| Titanium - 48 |
Physical Properties:
- Lustrous
- Ductile when pure
- Malleable when heated
- Silver color
- Low density
- Strong
- Not a good conductor of electricity
- Not a good conductor of heat
- Melting point : 1668 degrees Celsius (3034.4 Fahrenheit)
- Boiling point : 3287 degrees Celsius (5948.6 Fahrenheit)
How did Titanium get its Name
 |
| Titans of Greek Mythology |
Uses of Titanium
- Aircraft
- Spacecraft
- Cars
- Phone
- Building Purpose
- Dental Instruments
- Bone Structure
- Bicycle
- Squash Racket
Interesting Facts of Titanium
It took 119 years after Titanium was discovered just to isolate it into pure sample!!! It is never found in its pure form naturally, it can only be found bonded with other elements.
Health Effect
Overexposure to Titanium powder may cause tightness and pain in chest, coughing, difficulty in breathing. It will cause irritation when contact with eyes and skin.
THIS IS THE END OF TITANIUM
To go to homepage, click this link :
To go to Chemistry Element Page, click this link :
Thanks for visiting.
If there is any improvements needed, feel free to comment at the comment section below.
Thank you.
If there is any improvements needed, feel free to comment at the comment section below.
Thank you.


Comments
Post a Comment